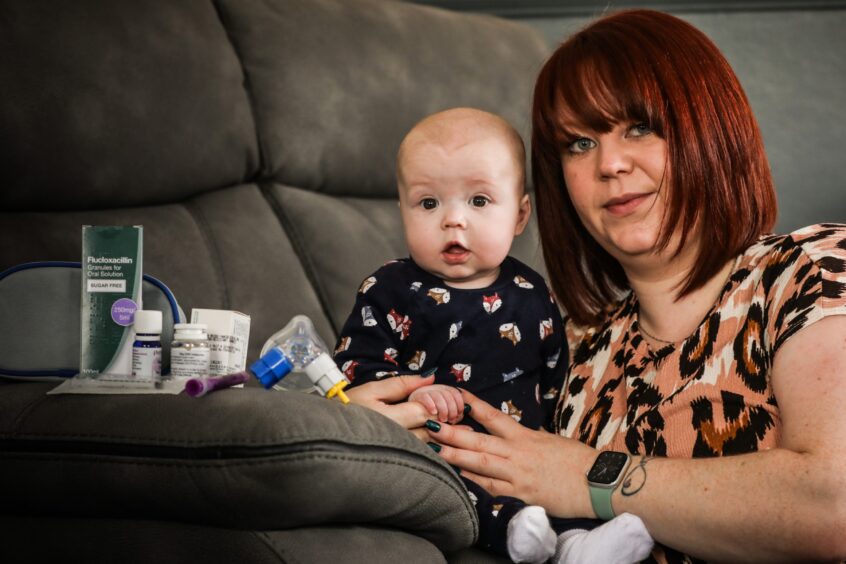
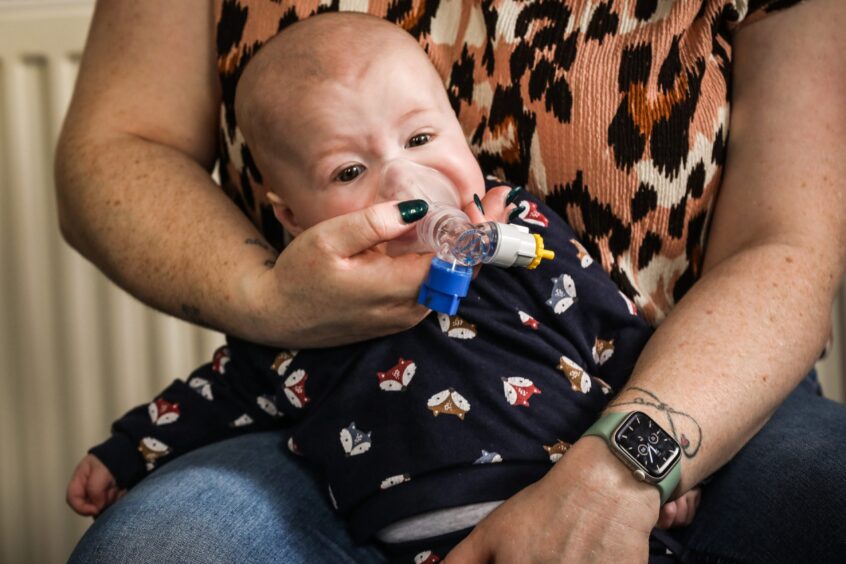
Angus mum Kirstin Adam fears her baby son could face a premature death from cystic fibrosis if the drugs he needs to survive are withdrawn from the NHS.
Kirstin, 31, and partner Andy Ashfield, 32, were devastated when they found out Arlo, had Cystic Fibrosis (CF) when he was just three-and-a-half weeks old.
Cystic fibrosis is a condition that causes sticky mucus to build up in the lungs and digestive system, meaning it can lead to a significantly shorter life expectancy for many sufferers.
But the couple pinned their hopes on new medication which they were told could help to prolong Arlo’s life into adulthood.
One of the drugs, Kaftrio, is licensed for children over the age of two, but it can cost £100,000 a year.
Now health bosses are consulting on whether it is too expensive to offer to new patients.
CF drugs ‘too costly’ to be NHS prescribed
Regulators are currently saying Kaftrio and two other drugs – Orkambi and Symkevi – are too costly to be prescribed on the NHS.
Kirstin said: “When we found out Arlo had cystic fibrosis, we were absolutely devastated.
“It came as such a shock.
“But we were completely reassured that he should be able to live a fairly normal, healthy life because of the breakthroughs in medication over the years.
“We were told it would be okay because our son had been born into an era like no other when it comes to cystic fibrosis – that there are amazing new treatments.
“Yet now we are hearing these drugs are too expensive to be prescribed on the NHS.”
Currently the drugs can continue to be prescribed for existing patients but new patients won’t be eligible, if the proposals under consultation are carried forward.
But Kirstin, who also has a nine-year-old daughter Amelia, said without them, many newborns and youngsters like her five-month-old son, might not be able to have a long and healthy life.
“It feels like we are going back in time essentially if they don’t change their stance on this.
“That this generation is going to have less of a life expectancy than generations that have come before, with the advances that have been made, just doesn’t make any sense.
‘Putting a price tag on my son’s life’
“My son could be looking at a premature death if he isn’t able to get access to Kaftrio and Orkambi which he is eligible for.
“You can’t put a price tag on someone’s life.”
Kirstin says health bosses are not looking at the ‘bigger picture’ as she says these drugs would stop patients with CF from needing hospital stays and extra antibiotics which would be costly in the long run.
She added: “Is doing this actually saving the NHS money by reducing a child’s life expectancy? It is like they are putting a price on my wee boy’s life.
“This is how I see it as a parent and it’s horrible.”
The move has prompted a number of parents across the country, whose children have cystic fibrosis, to urge health bosses to reconsider their initial decision, which is based on cost.
And a petition – which currently has over 43,000 signatures – has also been launched to help fund continued access to these drugs which could be a lifeline for people with cystic fibrosis.
David Ramsden is the CEO of the Cystic Fibrosis Trust.
He said the bodies that guide the NHS on which drugs it should prescribe had initially said Kaftrio, Orkambi and Symkevi were highly effective for people with cystic fibrosis.
The National Institute for Clinical Excellence (NICE) guides the NHS in England on available drugs while the Scottish Medicines Consortium (SMC) fulfils a similar role in Scotland.
Four-week consultation
NICE and SMC are currently holding a four-week consultation – which finishes this Friday – before deciding if the three drugs will continue to be offered to new patients.
Mr Ramsden said: “It is important to emphasise that those already taking any of the modulator drugs are not affected by the NICE process because of the agreements already in place.
“But this update creates uncertainty for those not yet on treatment.
“[Manufacturer] Vertex, NICE, and the NHS must now urgently work together to find a solution to make these treatments available for all those who could potentially benefit.
“We must never return to a situation where people with CF die far too young, knowing there’s a treatment that could change that.”
Working to deliver best outcome for people with cystic fibrosis
Helen Knight is director of medicines evaluation at NICE.
She said: “The committee want to hear from stakeholders through consultation on important aspects of its draft conclusions.
“This is to ensure that we have all the relevant information to accurately capture the value of these effective medicines when the committee makes its final decision.”
A spokesperson for the Scottish Medicines Consortium (SMC), said the draft recommendations could change following the consultation.
The spokesperson added: “Existing patients and new patients who are started on treatment while the evaluation is ongoing will continue to have access to the treatments after NICE has issued its final recommendations, irrespective of the outcome.”
The deadline for taking part in the NICE consultation is today (November 24) at 5pm.












Conversation